For portable electronic products, lithium-ion batteries are the most common choice. In contrast to other types of batteries, lithium-ion batteries are light in weight and do not have a memory effect. As compared to nickel-metal hydride batteries, lithium-ion batteries have twice as much energy density and have a 6-8 times lower self-discharge rate.
In order to ensure the safety of the application and optimize the use time of lithium-ion batteries, it is most important to understand their charging and discharging characteristics before designing an application.
Lithium-ion batteries are currently charged in three stages: precharge, constant current charging, and constant voltage charging.
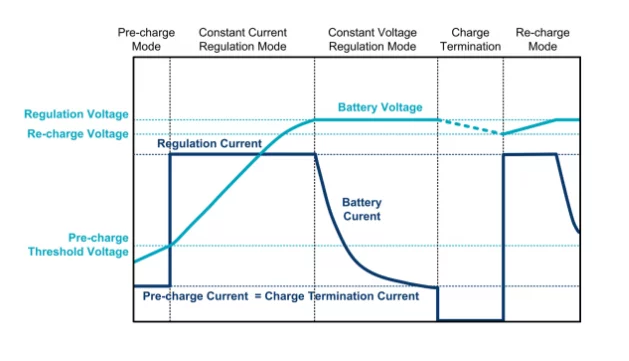
Why are there 3 stages?
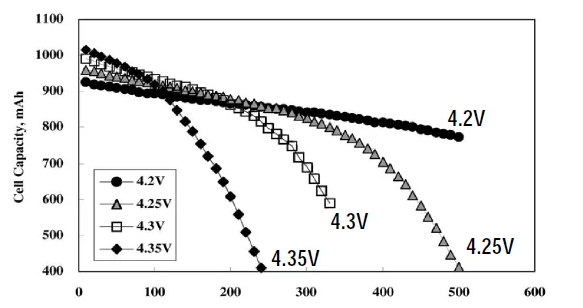
The figure below shows the relationship between the capacity, cycle life and charging voltage of lithium-ion batteries. The vertical axis is the battery capacity and the horizontal axis is the number of cycle lives. As can be seen from the above graph, the higher the charging cut-off voltage, the shorter the cycle life and the faster the capacity loss.
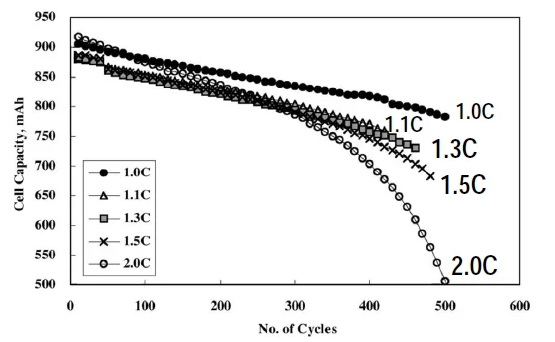
The figure below shows the relationship between the capacity, cycle life and discharge current of lithium-ion batteries. It is evident that the higher the charging rate, the faster the capacity attenuation speed will be. The vertical axis represents the battery capacity and the horizontal axis shows the number of cycle lives.
Chemical properties of lithium-ion batteries
Under the action of an external electric field provided by the charger, Li+ protrudes from the positive electrode LiCoO2 and moves to the negative electrode, composed of graphite. If the charging speed is too fast, it can prevent Li+ from entering the negative electrode lattice in a timely manner; these ions will instead accumulate near the negative electrode. When this happens, Li+ may capture electrons from the negative electrode and form metallic lithium. This gradual formation of lithium metal can cause dendritic crystal growth – commonly known as dendrites – near the negative electrode.
Furthermore, as the negative electrode is filled to a greater and greater extent, the LiC lattice becomes increasingly crowded and there are fewer and fewer spaces left, which decreases Li+’s ability to move from the positive electrode to find the space, as well as the duration of the movement. Continually charging at the same speed can lead to the accumulation of Li+ on the negative electrode’s surface.
A short circuit occurs when dendrites pierce the diaphragm between the positive and negative stages during the second half of charging.
It is clear that faster charging speeds, higher voltages, and longer charging durations can all be potentially dangerous. Consider the analogy of a soap bubble; if one were to inflate it too quickly with gas, the rate at which the water film expands may not keep pace with how fast the gas is entering, possibly resulting in bursting.
Under the action of an external electric field provided by the charger, Li+ protrudes from the positive electrode LiCoO2 and moves to the negative electrode, composed of graphite. If the charging speed is too fast, it can prevent Li+ from entering the negative electrode lattice in a timely manner; these ions will instead accumulate near the negative electrode. When this happens, Li+ may capture electrons from the negative electrode and form metallic lithium. This gradual formation of lithium metal can cause dendritic crystal growth – commonly known as dendrites – near the negative electrode.
A constant current charging current of about 1C is used (determined by the capacity attenuating to 80% of its initial capacity after 500 cycles of use).
It is believed that the battery is fully charged once the current drops to a certain level (usually C/10), at which point the charging process will be terminated.
The last stage is known as the supplementary stage, which is actually a combination of the constant current stage and the constant voltage stage. The purpose of this compensation measure is to make up for the decrease in capacitance caused by self-discharge and other loads connected to the battery. As a result, when the battery (and the system composed of it) is separated from the charging equipment, it is always fully charged.
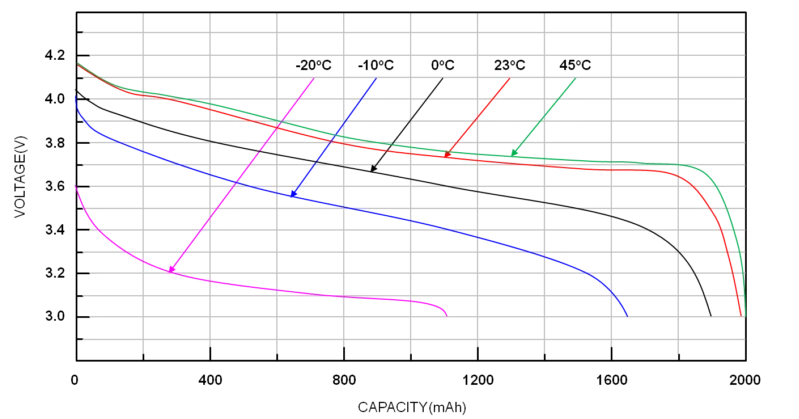
The charging strategy will also be heavily influenced by the temperature of the battery. As a result of the different characteristics of the materials that make up a battery at different temperatures, the battery’s capacity and charging voltage have also changed significantly over time.
Caution for the battery and the pack
In order to maintain battery safety and long-term performance of lithium rechargeable cells, the pack shall meet certain conditions.
Installing the battery into the pack
Before assembling the battery pack, the cell should be visually inspected.
It is not recommended to use damaged cells. (damaged surface, can distortion, electrolyte smell)
Packing cells with different Lot Numbers together is not recommended.
Use of different types of cells or the same types but made by different companies shouldn’t be combined.
Design of battery pack
Any charger other than the dedicated charger should not be used to charge the battery pack.
There is a feature in the battery pack that prevents external short cuts.
Charge
Constant Current-Constant Voltage (CC/CV) is the charging method.
During charging, the maximum voltage and current specified in the product specification should be used.
Charge the battery at the operating temperature specified in the product specification.
Discharge
In the case of using the battery for mobile equipment, the discharging method is Constant Current (CC).
The maximum discharge current specified in the product specification should be used for discharge.
The product specification specifies the cut off voltage for discharge.
It is recommended that the battery be discharged at the operating temperature specified in the product specification.